Product Description
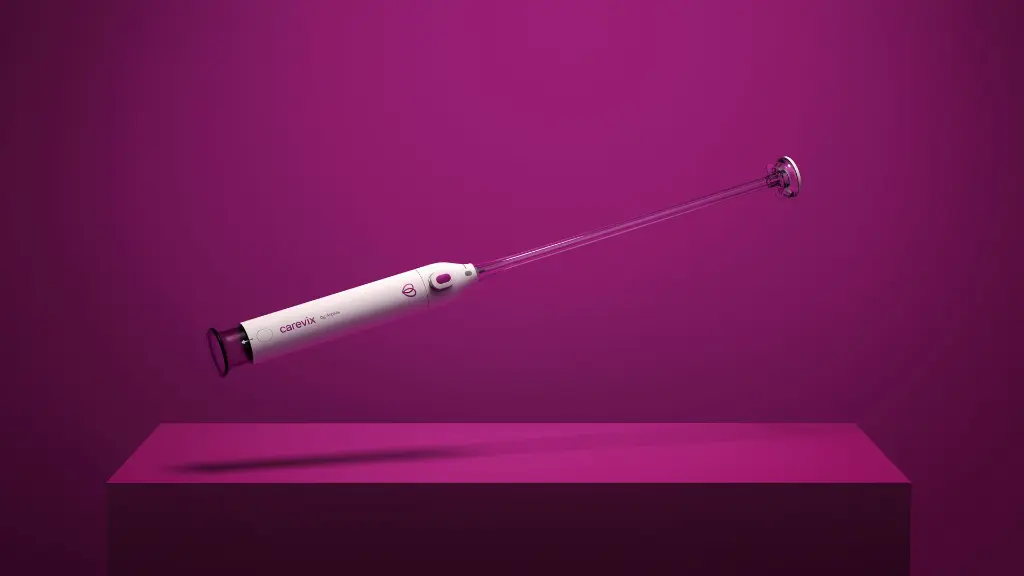
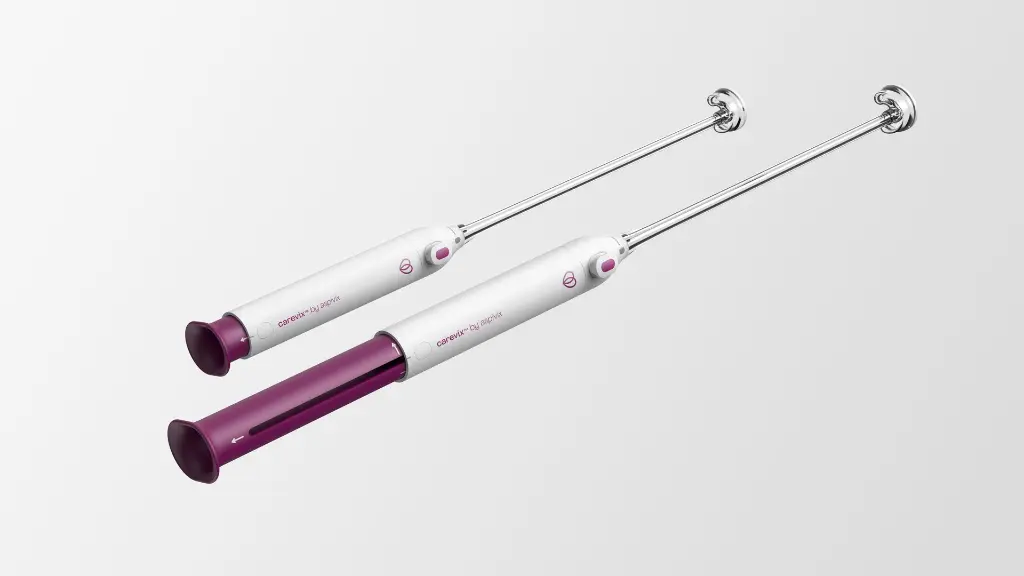
The carevix® Suction Cervical Stabilizer is a sterile, disposable, manual device designed for atraumatic snaring, grasping, holding and manipulation of cervical tissue during transcervical procedures.
It is a single-use, suction cervical stabilizer, engineered with vacuum technology, fully designed as a modern alternative to the Tenaculum without compromising on performance.
Intended Use and Contraindications
Please refer to our IFU for further information. Link to the electronic version is as follow: https://eifu.aspivix.com/.
Device Characteristics
Size (Device Without Packaging)
|
Device Dimensions (Assembled)
|
363mm X 26mm X 34mm
|
14.3in X 1.0in X 1.3in |
|
Device Weight (Without Packaging)
|
41g
|
1.45oz. |
Packaging (Device With Packaging)
|
Device Per Sterile Package
|
1 unit
|
|
|
Components In The Packaging
|
2 Components
|
|
|
Packaging Dimensions
|
330mm X 80mm X 30mm
|
13.0in X 3.1in X 1.2in |
|
Device Weight (With Packaging)
|
81g
|
2.86oz. |
Other Characteristics
|
Materials
|
Polycarbonate/Polyester alloy, MABS, and thermoplastic elastomer
|
Climate Contribution
Our product is certified with Climate Contribution
As part of its environmental strategy, Aspivix has decided to compensate all unavoidable emissions related to the production of carevix®. This strategy has resulted in an independent and verified product certification, validated by international standards.

How exactly does this work?
1. Calculation of our greenhouse gas emissions
Together with Climate Partner, Aspivix has measured the carbon emissions that occur during the manufacture of carevix® through raw materials, packaging, logistics and disposal: this is known as the Product Carbon Footprint.
2. Avoidance and reduction
Where possible, Aspivix avoids and reduces CO2 emissions. This can include specific logistic chains, developing products that will reduce existing emissions, or promoting public transportation as a mean for Aspivix' employees to come to the office.
3. Carbon offset through certified projects
Aspivix offsets all unavoidable CO2 emissions from carevix® by supporting carbon offset projects. Carbon offset projects save CO2 emissions - for example, through reforestation or the replacement of harmful technologies with climate-friendly alternatives.
4. The label "Climate Contribution"
Our product carevix® is certified with the independent label "Climate Contribution" , and consequently enables our customers to understand how the product has become carbon compensated and learn more about the carbon offset projects. This label therefore provides our customers with full transparency regarding the carbon contribution of our product.
Our carbon offset projects
To compensate for CO2 emissions that cannot be avoided, Aspivix supports recognised carbon offset projects, certified according to international standards. Further information can be found at: www.climatepartner.com/21055-2212-1001.